to remove an intron from mrna, what sites must be present?
What's the departure between mRNA and pre-mRNA? It's all about splicing of introns. Meet how one RNA sequence can exist in near 40,000 different forms.
For most eukaryotic genes (and some prokaryotic ones), the initial RNA that is transcribed from a gene's DNA template must be processed before it becomes a mature messenger RNA (mRNA) that can direct the synthesis of protein. 1 of the steps in this processing, called RNA splicing, involves the removal or "splicing out" of certain sequences referred to as intervening sequences, or introns. The final mRNA thus consists of the remaining sequences, chosen exons, which are connected to one another through the splicing process. RNA splicing was initially discovered in the 1970s, overturning years of thought in the field of cistron expression.
Early on Studies in Leaner
Cistron regulation was showtime studied most thoroughly in relatively elementary bacterial systems. About bacterial RNA transcripts do not undergo splicing; these transcripts are said to be colinear, with Dna directly encoding them. In other words, there is a ane-to-one correspondence of bases between the cistron and the mRNA transcribed from the cistron (excepting v′ and 3′ noncoding regions). However, in 1977, several groups of researchers who were working with adenoviruses that infect and replicate in mammalian cells obtained some surprising results. These scientists identified a series of RNA molecules that they termed "mosaics," each of which contained sequences from noncontiguous sites in the viral genome (Berget et al., 1977; Chow et al., 1977). These mosaics were establish late in viral infection. Studies of early infection revealed long primary RNA transcripts that contained all of the sequences from the tardily RNAs, every bit well equally what came to exist called the intervening sequences (introns).
Subsequent to the adenoviral discovery, introns were constitute in many other viral and eukaryotic genes, including those for hemoglobin and immunoglobulin (Darnell, 1978). Splicing of RNA transcripts was then observed in several in vitro systems derived from eukaryotic cells, including removal of introns from transfer RNA in yeast cell-gratuitous extracts (Knapp et al., 1978). These observations solidified the hypothesis that splicing of large initial transcripts did, in fact, yield the mature mRNA. Other hypotheses proposed that the Dna template in some way looped or assumed a secondary structure that immune transcription from noncontiguous regions (Darnell, 1978).
How Splicing Occurs
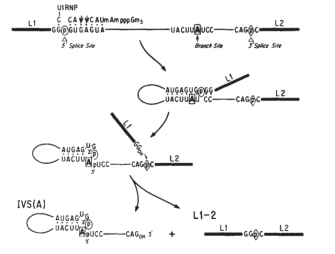
© 1985 Nature Publishing Group Konarska, M. M. et al. Characterization of the branch site in lariat RNAs produced by splicing of mRNA precursors. Nature 313, 556 (1985). All rights reserved. ![]()
The biochemical mechanism by which splicing occurs has been studied in a number of systems and is now fairly well characterized. Introns are removed from primary transcripts past cleavage at conserved sequences called splice sites. These sites are plant at the 5′ and 3′ ends of introns. Nigh unremarkably, the RNA sequence that is removed begins with the dinucleotide GU at its v′ finish, and ends with AG at its three′ end. These consensus sequences are known to be disquisitional, because changing one of the conserved nucleotides results in inhibition of splicing. Some other of import sequence occurs at what is chosen the co-operative bespeak, located anywhere from 18 to 40 nucleotides upstream from the 3′ end of an intron. The branch signal e'er contains an adenine, but it is otherwise loosely conserved. A typical sequence is YNYYRAY, where Y indicates a pyrimidine, North denotes any nucleotide, R denotes any purine, and A denotes adenine. Rarely, alternate splice site sequences are found that brainstorm with the dinucleotide AU and finish with Ac; these are spliced through a like mechanism.
Splicing occurs in several steps and is catalyzed by modest nuclear ribonucleoproteins (snRNPs, commonly pronounced "snurps"). Showtime, the pre-mRNA is cleaved at the 5′ end of the intron following the zipper of a snRNP chosen U1 to its complementary sequence within the intron. The cut end then attaches to the conserved branch point region downstream through pairing of guanine and adenine nucleotides from the 5′ end and the branch point, respectively, to class a looped structure known as a lariat (Figure 1). The bonding of the guanine and adenine bases takes place via a chemical reaction known as transesterification, in which a hydroxyl (OH) grouping on a carbon cantlet of the adenine "attacks" the bond of the guanine nucleotide at the splice site. The guanine residue is thus cleaved from the RNA strand and forms a new bond with the adenine.
Adjacent, the snRNPs U2 and U4/U6 appear to contribute to positioning of the 5′ stop and the branch point in proximity. With the participation of U5, the 3′ end of the intron is brought into proximity, cut, and joined to the 5′ end. This step occurs by transesterification; in this instance, an OH group at the 3′ end of the exon attacks the phosphodiester bond at the 3′ splice site. The adjoining exons are covalently bound, and the resulting lariat is released with U2, U5, and U6 bound to it.
In improver to consensus sequences at their splice sites, eukaryotic genes with long introns too contain exonic splicing enhancers (ESEs). These sequences, which assistance position the splicing apparatus, are found in the exons of genes and bind proteins that aid recruit splicing mechanism to the right site. Well-nigh splicing occurs between exons on a unmarried RNA transcript, but occasionally trans-splicing occurs, in which exons on different pre-mRNAs are ligated together.
The splicing process occurs in cellular machines called spliceosomes, in which the snRNPs are constitute along with boosted proteins. The primary variety of spliceosome is one of the most plentiful structures in the cell, and recently, a secondary blazon of spliceosome has been identified that processes a minor category of introns. These introns are referred to as U12-type introns because they depend upon the action of a snRNP called U12 (the common introns described higher up are called U2-blazon introns). The function of U12-blazon introns is non yet defined, just their persistence throughout evolution and conservation between homologous genes of widely divergent species suggests an important functional basis (Patel & Steitz, 2003).
Self-Splicing and Alternative Splicing
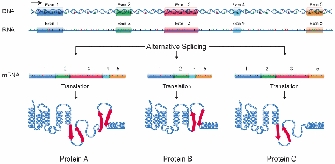
Copyright National Institutes of Health
Some RNA molecules have the capacity to splice themselves; the initial discovery of this self-splicing ability in the protozoan Tetrahymena thermophila was recognized with the Nobel Prize in 1989. The self-splicing introns establish in T. thermophila are now referred to as Group I introns; this course also includes other protozoan ribosomal RNA genes, some fungal mitochondrial genes, and some phage genes. Group I introns all fold into a complex secondary structure with 9 loops and employ transesterification reactions equally described in a higher place. On the other hand, Grouping II self-splicing introns are found in mitochondrial genes and are excised by a mechanism that bears similarities to pre-mRNA splicing, including the production of lariats. For this reason, it has been proposed that perhaps pre-mRNA introns and splicing mechanisms evolved from the Group Ii introns.
Early in the class of splicing research, yet another surprising discovery was made; specifically, researchers noticed that non only was pre-mRNA punctuated by introns that needed to be excised, only besides that alternative patterns of splicing inside a unmarried pre-mRNA molecule could yield different functional mRNAs (Figure 2; Berget et al. 1977). The first example of alternative splicing was defined in the adenovirus in 1977 and demonstrated that one pre-mRNA molecule could be spliced at different junctions to result in a diversity of mature mRNA molecules, each containing dissimilar combinations of exons.
Before long afterward, culling splicing was institute to occur in cellular genes as well, with the starting time example identified in the IgM gene, a member of the immunoglobulin superfamily (Early et al., 1980). Another example of a gene with an impressive number of alternative splicing patterns is the Dscam gene from Drosophila, which is involved in guiding embryonic nerves to their targets during germination of the wing's nervous organization. Examination of the Dscam sequence reveals such a large number of introns that differential splicing could, in theory, create a staggering 38,000 different mRNAs. This ability to create so many mRNAs may provide the diversity necessary for forming a complex construction such as the nervous system (Schmucker et al., 2000). In fact, the existence of multiple mRNA transcripts within single genes may account for the complexity of some organisms, such every bit humans, that accept relatively few genes (approximately 20,000). For example, work from Wang et al. (2008) suggests that more ninety% of homo genes are alternatively spliced.
The By and Hereafter of Introns
The existence of introns and differential splicing helps explain how new genes are created during evolution. Splicing makes genes more "modular," allowing new combinations of exons to be created during evolution. Furthermore, new exons can exist inserted into old introns, creating new proteins without disrupting the function of the onetime cistron.
Our knowledge of RNA splicing is quite new. Yet, because well-nigh all eukaryotes have introns and share mechanisms of RNA splicing, splicing itself must be quite ancient. Proponents of the "intron-early" theory suggest that all organisms (including prokaryotes) at once had introns in their genome merely subsequently lost these elements, while "intron-late" supporters believe that the restriction of introns to eukaryotes suggests a more recent introduction (Roy & Gilbert, 2006). There is no apparent pattern in which eukaryotes take introns, and that makes information technology difficult for researchers to make predictions about how introns were gained or lost through development. What is articulate, still, is that introns and splicing have clearly played a pregnant role in evolution, and scientists are only beginning to observe the nature of that role.
References and Recommended Reading
Berget, S. M., et al. Spliced segments at the 5' terminus of adenovirus ii late mRNA. Proceedings of the National Academy of Sciences 74, 3171–3175 (1977)
Chow, L. T., et al. An amazing sequence arrangement at the v′ ends of adenovirus two messenger RNA. Cell 12, 1–8 (1977)
Darnell, J. E., Jr. Implications of RNA–RNA splicing in evolution of eukaryotic cells. Science 202, 1257–1260 (1978) doi:10.1126/science.364651
Early on, P., et al. Two mRNAs tin be produced from a single immunoglobulin chain past alternative RNA processing pathways. Prison cell 20, 313–319 (1980)
Knapp, G., et al. Transcription and processing of intervening sequences in yeast tRNA genes. Cell 14, 221–236 (1978)
Konarska, M. G., et al. Characterization of the branch site in lariat RNAs produced by splicing of mRNA precursors. Nature 313, 552–557 (1984) doi: x.1038/313552a0 (link to article)
Patel, A. A., & Steitz, J. A. Splicing double: Insights from the 2d spliceosome. Nature iv, 960–970 (2003) doi:10.1038/nrm1259 (link to commodity)
Pierce, B. A. Genetics: A Conceptual Approach, 2nd ed. (New York, Freeman, 2000)
Roy, Southward. W., & Gilbert, W. The evolution of spliceosomal introns: Patterns, puzzles, and progress. Nature Reviews Genetics seven, 211–221 (2006) doi: 10.1038/nrg1807 (link to article)
Schmucker, D., et al. Drosophila Dscam is an axon guidance receptor exhibiting boggling molecular diversity. Cell 101, 671–684 (2000)
Source: http://www.nature.com/scitable/topicpage/rna-splicing-introns-exons-and-spliceosome-12375#:~:text=Introns%20are%20removed%20from%20primary,and%203%E2%80%B2%20ends%20of%20introns.
0 Response to "to remove an intron from mrna, what sites must be present?"
Post a Comment